 Researchers from Massachusetts Institute of Technology led by Professor Daniel Nocera have produced an artificial leaf, a device that can turn the energy of sunlight directly into a chemical fuel which can be stored and used as energy source later.
Researchers from Massachusetts Institute of Technology led by Professor Daniel Nocera have produced an artificial leaf, a device that can turn the energy of sunlight directly into a chemical fuel which can be stored and used as energy source later.
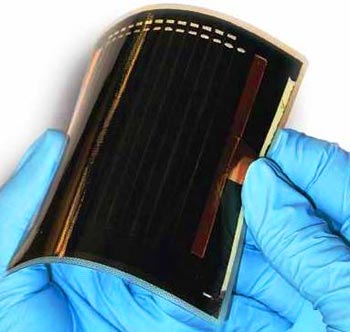
The artificial leaf is a silicon solar cell made by different catalytic materials bounded onto its two sides using no external wires or other control circuits to operate. Professor Nocera explains that this device is made entirely of earth-abundant, inexpensive materials such as silicon, cobalt and nickel. Other attempts to design a device like this before have relied on corrosive solutions or on relatively rare and expensive materials such as platinum.
Placing the artificial leaf into a container of ordinary water and exposed to sunlight, it quickly begins to generate streams of bubbles: on the one side containing oxygen and on the other side hydrogen.
“You can’t get more portable — you don’t need wires, it’s lightweight,” Nyocera says and it doesn’t require much in the way of additional equipment, other than a way of catching and storing the gases that bubble off. “You just drop it in a glass of water, and it starts splitting it,” he says.
The artificial leaf is not yet ready for commercial production, since systems to collect, store and use the gases remain to be developed. “It’s a step,” Nocera says. “It’s heading in the right direction.”
Ultimately, he sees a future in which individual homes could be equipped with solar-collection systems based on this principle: Panels on the roof could use sunlight to produce hydrogen and oxygen that would be stored in tanks, and then fed to a fuel cell whenever electricity is needed. Such systems, Nocera hopes, could be made simple and inexpensive enough so that they could be widely adopted throughout the world, including many areas that do not presently have access to reliable sources of electricity.
Nocera’s ongoing research with the artificial leaf is directed toward “driving costs lower and lower,” he says, and looking at ways of improving the system’s efficiency. At present, the leaf can redirect about 2.5 percent of the energy of sunlight into hydrogen production in its wireless form; a variation using wires to connect the catalysts to the solar cell rather than bonding them together has attained 4.7 percent efficiency of ordinary 10 percent for a commercial solar cell today.
Another line of research is to explore the use of photovoltaic, solar cell, materials other than silicon — such as iron oxide, which might be even cheaper to produce. “It’s all about providing options for how you go about this,” Nocera added.



