A low cost catalysts transform carbon dioxide gas into valuable building blocks for organic synthesis
Chemists are helping to reduce heat-trapping carbon dioxide emissions, which are a global concern.
For example, they are devising new catalytic systems that would enable waste carbon dioxide to be recycled as a non-toxic and practically free source of carbon for organic synthetic reactions. However, current carbon dioxide conversion techniques require expensive metal catalysts or drawn-out procedures.
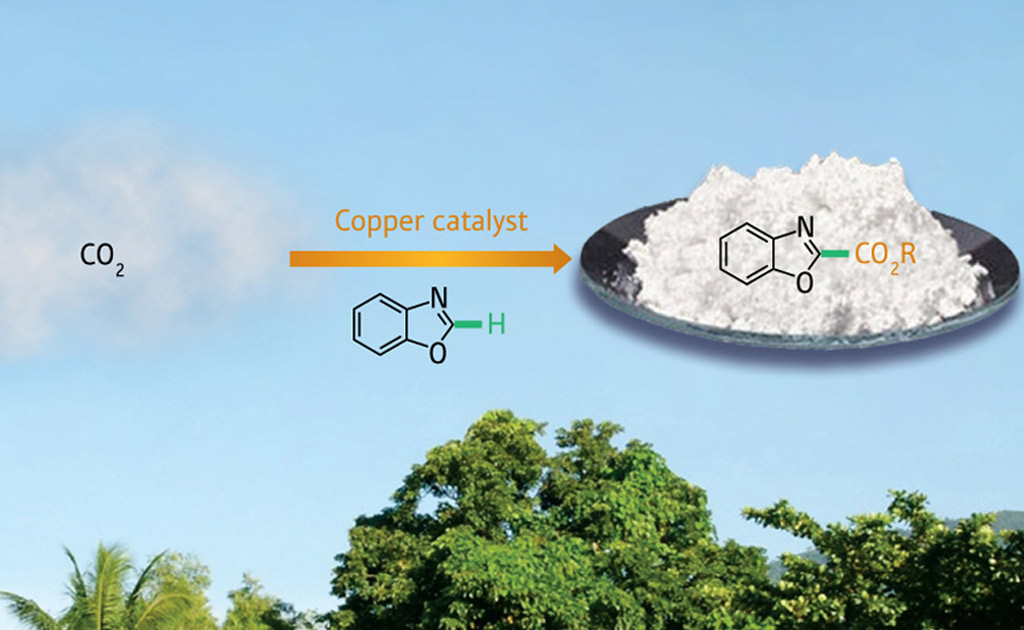
Now, Zhaomin Hou and colleagues from the RIKEN Advanced Science Institute in Wako have found a way to insert carbon dioxide directly into the framework of aromatic molecules, turning them into carboxylic acid derivatives that are widely used as pharmaceuticals, agrichemicals, and dyes. Importantly, this transformation can be achieved economically and with negligible environmental impact, thanks to a low cost copper complex bearing an organic ligand.
N-heterocyclic carbenes (NHCs) are molecules with near metal-like reactivity because of an electron-deficient carbon center. For the past two decades, scientists have used NHCs as organic replacements for metal catalysts and as ‘spectator’ ligands that attach to metal centers and influence their catalytic behavior. Hou and colleagues recently discovered that adding NHCs to copper, one of the most abundant metals in nature, created a complex that catalyzed carbon dioxide addition to boron esters—a trick the team hoped to repeat with aromatic hydrocarbons.
The most efficient way to incorporate carbon dioxide into benzene-like molecules is by replacing one of the carbon–hydrogen (C–H) bonds on the outer ring; unfortunately, these bonds are notoriously unreactive. To overcome this problem, the researchers turned to benzoxazole: this double-ringed aromatic compound has a C–H bond situated between nitrogen and oxygen atoms, making it easier to chemically activate this position.
With just a pinch of the NHC–copper catalyst complex, the team found they could convert a mixture of carbon dioxide and several different benzoxazole-based molecules into solid carboxylic acids and esters in excellent yields . Carefully characterizing the crystal structures of several intermediate compounds revealed that carbon dioxide inserted in between a copper–carbon bond formed at the benzoxazole C–H site, followed by a dissociation step that regenerated the catalyst.
According to Hou, the NHC ligand was essential in enabling carbon dioxide capture. “The electron-donating ability of NHC could make the C–H activation and carbon dioxide insertion steps easier, while its steric bulk brings stability to the active catalyst species,” he notes. The researchers now hope to extend this technique to less reactive C–H bonds by fine-tuning the catalyst complex and optimizing reaction conditions.



