For the first time, physical chemistry reactions in a fuel cell can now be observed and described in detail on the nanoscale.
This innovation is due to a new microscopy technique devised by an international research team involving Heidelberg mathematician Dr. Francesco Ciucci and scientists from the United States and Ukraine. The new technique means that oxygen reduction, which is significant for the energy generated in a fuel cell, can now be represented at a resolution of one millionth of a millimeter. The research findings will be used to develop more efficient and powerful hydrogen fuel cells.
Fuel cells convert the energy in a fuel like hydrogen into electric energy. A hydrogen fuel cell consists of two electrodes facing one another and separated by an ion conductor. Electric energy is gained via transfer of ions between the two electrodes.
The oxygen in the air reacts with the hydrogen brought in from outside. In this process of oxygen reduction, a catalyst – frequently the rare and expensive platinum – plays an essential role as reaction accelerator. In all this, says Francesco Ciucci, the oxygen reduction process is the limiting factor in connection with the longevity and efficiency of fuel cells.
“To optimise ion transfer between the electrodes, a number of fundamental questions have to be answered,” says the mathematician. “How and where exactly does oxygen reduction occur and how does platinum function as a catalyst? Up to now we have had to do without a suitable instrument for investigating the reaction dynamics involved.”
Francesco Ciucci’s research has been funded by a grant from Heidelberg University’s Graduate School of Mathematical and Computational Methods for the Sciences. In its course, and in conjunction with Dr. Amit Kumar at the Oak Ridge National Laboratory in the USA and Dr. Anna Morozovska of the Ukrainian National Academy of Sciences, Dr. Ciucci has developed a new microscopy technique that can monitor ion transfer on the nanoscale. The technique is called “Electrochemical Strain Microscopy” (ESM).
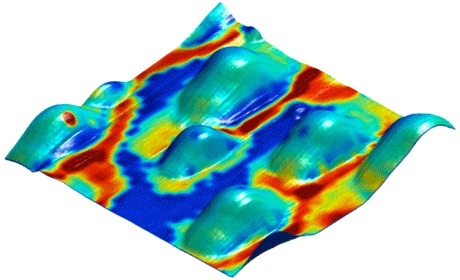
The ESM technique is based on a mathematical model, a so-called partial differential equation, that describes the movement of oxygen in various materials.
By way of this mathematical description, the measurement data from “Electrochemical Strain Microscopy” could be visualised on the computer screen. “What we have found out from this,” says Dr. Ciucci, “is that the catalyst layer of 50-nanometre platinum particles does not allow an equal degree of ion transfer at all points.” The innovative microscopy technique ESM is not restricted to the optimisation of fuel cells. Dr. Ciucci points out that it is suitable for investigating chemical processes on all surfaces where materials interact via ion transfer.



