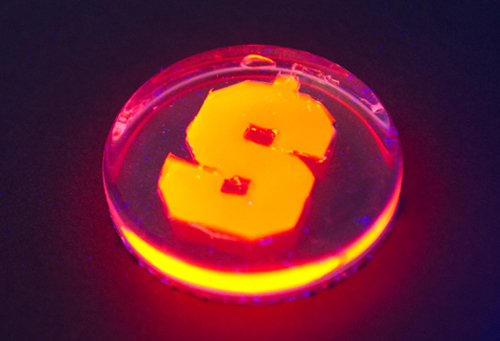
What do fireflies, nanorods, and Christmas lights have in common? Someday, consumers may be able to purchase multicolor strings of light that don’t need electricity or batteries to glow.
Scientists at Syracuse University found a new way to harness the natural light produced by fireflies (called bioluminescence) using nanoscience. Their breakthrough produces a system that is 20 to 30 times more efficient than those produced during previous experiments.
It’s all about the size and structure of the custom, quantum nanorods, which are produced in the laboratory by Mathew Maye, assistant professor of chemistry in SU’s College of Arts and Sciences; and Rebeka Alam, a chemistry Ph.D. candidate. Maye is also a member of the Syracuse Biomaterials Institute.
“Firefly light is one of nature’s best examples of bioluminescence,” Maye says. “The light is extremely bright and efficient. We’ve found a new way to harness biology for non-biological applications by manipulating the interface between the biological and non-biological components.”
Their work, “Designing Quantum Rods for Optimized Energy Transfer with Firefly Luciferase Enzymes,” was published online May 23 in Nano Letters and is forthcoming in print. Nano Letters is a premier journal of the American Chemical Society and one of the highest rated journals in the nanoscience field. Collaborating on the research were Professor Bruce Branchini and Danielle Fontaine, both from Connecticut College.
Fireflies produce light through a chemical reaction between luciferin and it’s counterpart, the enzyme luciferase. In Maye’s laboratory, the enzyme is attached to the nanorod’s surface; luciferin, which is added later, serves as the fuel. The energy that is released when the fuel and the enzyme interact is transferred to the nanorods, causing them to glow. The process is called Bioluminescence Resonance Energy Transfer (BRET).
“The trick to increasing the efficiency of the system is to decrease the distance between the enzyme and the surface of the rod and to optimize the rod’s architecture,” Maye says. “We designed a way to chemically attach, genetically manipulated luciferase enzymes directly to the surface of the nanorod.” Maye’s collaborators at Connecticut College provided the genetically manipulated luciferase enzyme.
The nanorods are composed of an outer shell of cadmium sulfide and an inner core of cadmium seleneide. Both are semiconductor metals. Manipulating the size of the core, and the length of the rod, alters the color of the light that is produced. The colors produced in the laboratory are not possible for fireflies. Maye’s nanorods glow green, orange, and red. Fireflies naturally emit a yellowish glow. The efficiency of the system is measured on a BRET scale. The researchers found their most efficient rods (BRET scale of 44) occurred for a special rod architecture (called rod-in-rod) that emitted light in the near-infrared light range. Infrared light has longer wavelengths than visible light and is invisible to the eye. Infrared illumination is important for such things as night vision goggles, telescopes, cameras, and medical imaging.
Maye’s and Alam’s firefly-conjugated nanorods currently exist only in their chemistry laboratory. Additional research is ongoing to develop methods of sustaining the chemical reaction—and energy transfer—for longer periods of time and to “scale-up” the system. Maye believes the system holds the most promise for future technologies that that will convert chemical energy directly to light; however, the idea of glowing nanorods substituting for LED lights is not the stuff of science fiction.
“The nanorods are made of the same materials used in computer chips, solar panels, and LED lights,” Maye says. “It’s conceivable that someday firefly-coated nanorods could be inserted into LED-type lights that you don’t have to plug in.”
Maye’s research was funded by a Department of Defense PECASE award sponsored by the Air Force Office of Scientific Research (AFOSR). The AFOSR and the National Science Foundation supported the work performed by Maye’s collaborators at Connecticut College.