Researchers at the Department of Energy’s Oak Ridge National Laboratory have developed a biohybrid photo-conversion system — based on the interaction of photosynthetic plant proteins with synthetic polymers – that can convert visible light into hydrogen fuel.
Photosynthesis, the natural process carried out by plants, algae and some bacterial species, converts sunlight energy into chemical energy and sustains much of the life on earth. Researchers have long sought inspiration from photosynthesis to develop new materials to harness the sun’s energy for electricity and fuel production.
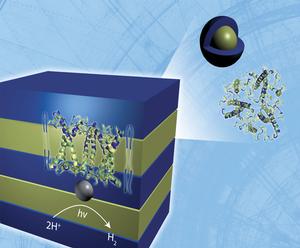
In a step toward synthetic solar conversion systems, the ORNL researchers have demonstrated and confirmed with small-angle neutron scattering analysis that light harvesting complex II (LHC-II) proteins can self-assemble with polymers into a synthetic membrane structure and produce hydrogen.
The researchers envision energy-producing photoconversion systems similar to photovoltaic cells that generate hydrogen fuel, comparable to the way plants and other photosynthetic organisms convert light to energy.
“Making a, self-repairing synthetic photoconversion system is a pretty tall order. The ability to control structure and order in these materials for self-repair is of interest because, as the system degrades, it loses its effectiveness,” ORNL researcher Hugh O’Neill, of the lab’s Center for Structural Molecular Biology, said.
“This is the first example of a protein altering the phase behavior of a synthetic polymer that we have found in the literature. This finding could be exploited for the introduction of self-repair mechanisms in future solar conversion systems,” he said.
Small angle neutron scattering analysis performed at ORNL’s High Flux Isotope Reactor (HFIR) showed that the LHC-II, when introduced into a liquid environment that contained polymers, interacted with polymers to form lamellar sheets similar to those found in natural photosynthetic membranes.
The ability of LHC-II to force the assembly of structural polymers into an ordered, layered state – instead of languishing in an ineffectual mush – could make possible the development of biohybrid photoconversion systems. These systems would consist of high surface area, light-collecting panes that use the proteins combined with a catalyst such as platinum to convert the sunlight into hydrogen, which could be used for fuel.
The research builds on previous ORNL investigations into the energy-conversion capabilities of platinized photosystem I complexes – and how synthetic systems based on plant biochemistry can become part of the solution to the global energy challenge.
“We’re building on the photosynthesis research to explore the development of self-assembly in biohybrid systems. The neutron studies give us direct evidence that this is occurring,” O’Neill said.
The researchers confirmed the proteins’ structural behavior through analysis with HFIR’s Bio-SANS, a small-angle neutron scattering instrument specifically designed for analysis of biomolecular materials.
“Cold source” neutrons, in which energy is removed by passing them through cryogenically chilled hydrogen, are ideal for studying the molecular structures of biological tissue and polymers.
The LHC-II protein for the experiment was derived from a simple source: spinach procured from a local produce section, then processed to separate the LHC-II proteins from other cellular components. Eventually, the protein could be synthetically produced and optimized to respond to light.
O’Neill said the primary role of the LHC-II protein is as a solar collector, absorbing sunlight and transferring it to the photosynthetic reaction centers, maximizing their output. “However, this study shows that LHC-II can also carry out electron transfer reactions, a role not known to occur in vivo,” he said.
The research team, which came from various laboratory organizations including its Chemical Sciences Division, Neutron Scattering Sciences Division, the Center for Structural Molecular Biology and the Center for Nanophase Materials Sciences, consisted of O’Neill, William T. Heller, and Kunlun Hong, all of ORNL; Dimitry Smolensky of the University of Tennessee; and Mateus Cardoso, a former postdoctoral researcher at ORNL now of the Laboratio Nacional de Luz Sincrotron in Brazil.
“That’s one of the nice things about working at a national laboratory. Expertise is available from a variety of organizations,” O’Neill said.
The work, published in the journal Energy & Environmental Science, was supported with Laboratory-Directed Research and Development funding. HFIR is supported by the DOE Office of Science.



