The design of today’s rechargeable lithium ion batteries limits the use of new technologies like electric cars and grid-scale energy storage because they do not store enough energy relative to their volume and weight – or, as researchers would say, their energy density is too low.
Stanford researchers have used nanotechnology to invent a better lithium ion battery cathode.
Solving that problem is largely a matter of finding new materials for the positively and negatively charged battery electrodes, the cathode and anode.
The research group of battery inventor Yi Cui, an associate professor of materials science and engineering, uses nanotechnology to fabricate electrode materials that greatly improve the electrical storage capacity of lithium ion batteries. In previous research, they reinvented battery anodes by fabricating them with silicon nanowires.
Now, Cui and his students have used sulfur-coated hollow carbon nanofibers and a special electrolyte additive to improve the other end of the rechargeable lithium ion battery, the cathode.
According to Cui, putting silicon nanowire anodes and sulfur-coated carbon cathodes into one battery is the next generation of battery design.
“I strongly believe that’s a promising future choice to make better batteries,” Cui said.
“Sulfur is one of the materials that can offer a 10-times higher charge storage capacity but with about half the voltage of the existing battery,” he said.
Both the charge capacity and the voltage affect how much energy a battery can deliver. With the sulfur cathode as part of a complete battery, the higher charge capacity makes it possible to build a battery with four to five times the energy storage compared to existing lithium ion battery technology.
Lithium-sulfur batteries have received attention because of the low cost and non-toxicity of sulfur. However, previous generations of lithium sulfur cathodes have not been viable for commercialization because they rapidly fail from repeated charging and recharging.
The new cathode fabrication resolves a number of material issues that, Cui said, “added together represent a really big challenge to get this material to work as a viable battery.”
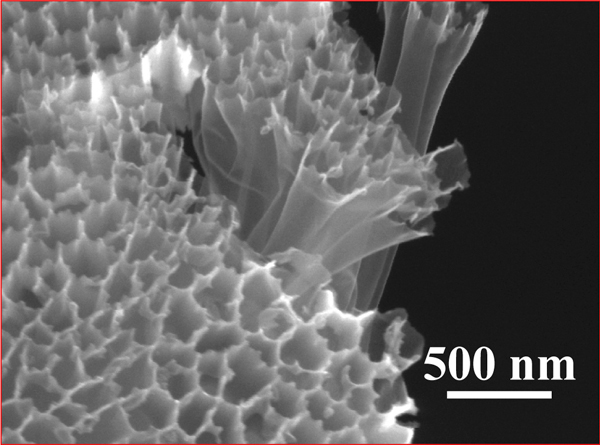
In previous lithium-sulfur cathode designs, sulfur coats onto relatively open carbon structures. This is a problem because it exposes sulfur to the battery’s electrolyte solution. When intermediate reaction products called lithium polysulfides come into contact with the electrolyte solution, they reduce the battery’s capacity by dissolving into the electrolyte.
As Cui’s graduate student, Wesley Guangyuan Zheng, explained, “This can be conflicting because on the one side we don’t want a large surface area contacting the sulfur and the electrolyte, and on the other hand we want a large surface area for electrical and ionic conductivities.”
The new design solves the conflict with a unique fabrication process that allows sulfur to coat the inside of a hollow carbon nanofiber, but not the outside. This fabrication process relies on a novel use of a commercially available filter technology that is normally applied to water filtration.
The new cathode design also improves battery capacity because it has a nearly closed structure that prevents polysulfides from significantly leaking out into the electrolyte solution. The length of a hollow nanofiber is about 300 times its diameter; the long and narrow channels prevent polysulfides from leaking out.
In addition to the energy storage gains made with improved sulfur hollow carbon nanofiber fabrication, Cui’s graduate student Yuan Yang included an electrolyte additive that enhances the battery’s charge and energy efficiency, known as the coulombic efficiency.
“Without the additive you put 100 electrons into the battery and you get 85 out. With the additive, you get 99 out,” Cui said.
“To design the best structure we need both the electrode design and the electrolyte additive and these two combined together can give you a high capacity and high coulombic efficiency,” Cui said. “We now have high capacity on both sides of the electrode; that’s exciting.”